
TermoSalud has achieved its third publication in an international scientific journal, the spotlight is once again on the Symmed ELITE Aesthetic, with study focused on body contouring treatments and improving the appearance of the skin on the abdomen and flanks. The article has been published in Lasers in Surgery and Medicine, the official journal of the American Society for Laser Medicine and Surgery, one of the most prestigious publications in the field.
Body Shaping and Skin Appearance Improvement in the Abdomen and Flanks by Radiofrequency Technology
This study aims to assess the safety and effectiveness of the Symmed radiofrequency (RF)-based device (Termosalud Inc., Gijón, Spain) in body contouring and skin appearance improvement treatments for abdomen/flanks.
Overview
Material and Methods
Eight sessions of Symmed RF were performed in 15 volunteers’ abdomen/flanks. Sessions were conducted every 72/96 h. To evaluate the efficacy and safety of the device, photographs, body contours, and ultrasound scans of dermal echogenicity, and adipose tissue thickness were taken at baseline and after the treatments. Additionally, a blind evaluation of the photographic results was conducted. Data regarding the satisfaction level of the participants was also obtained through a customized survey.
Results
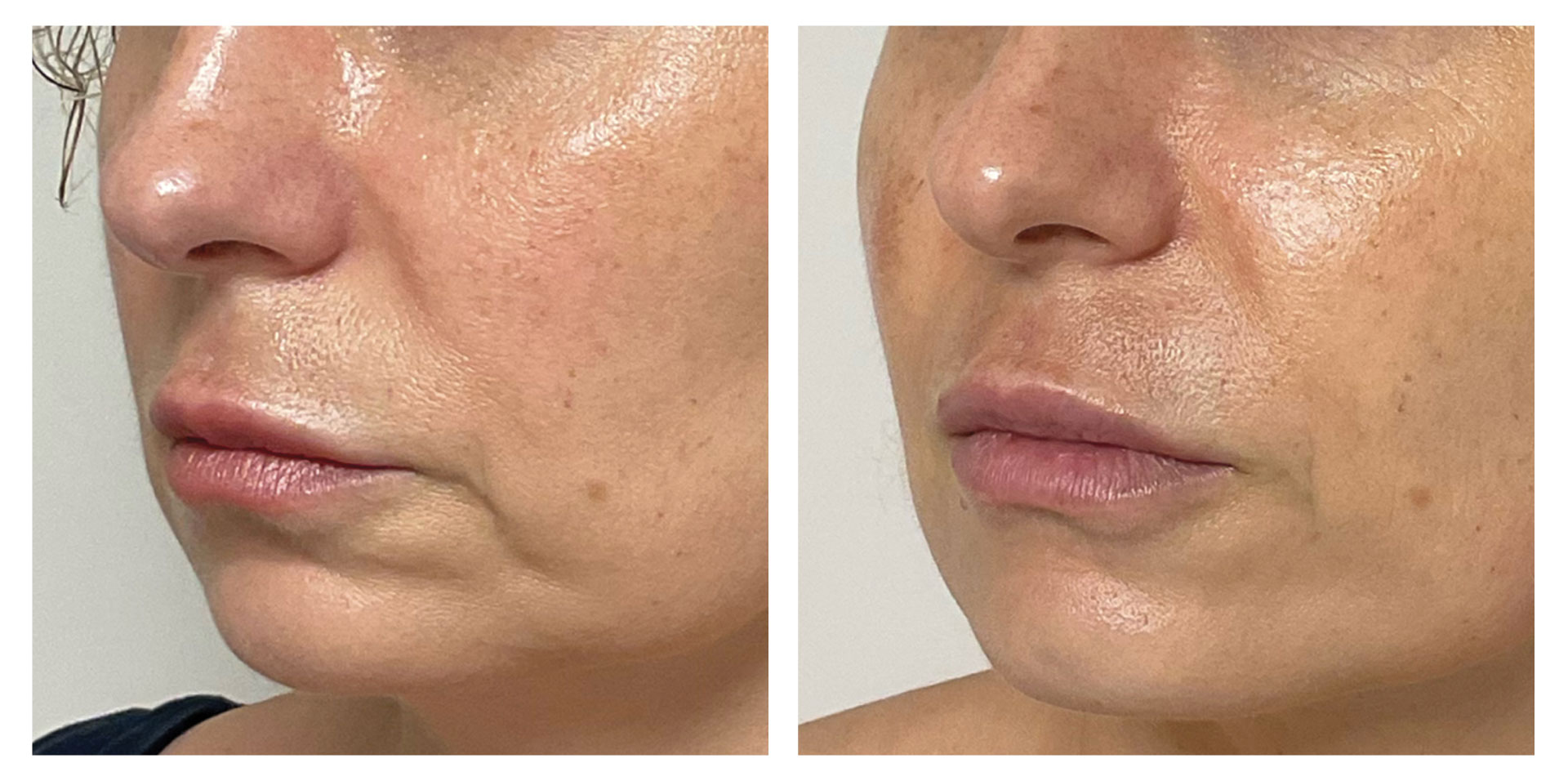
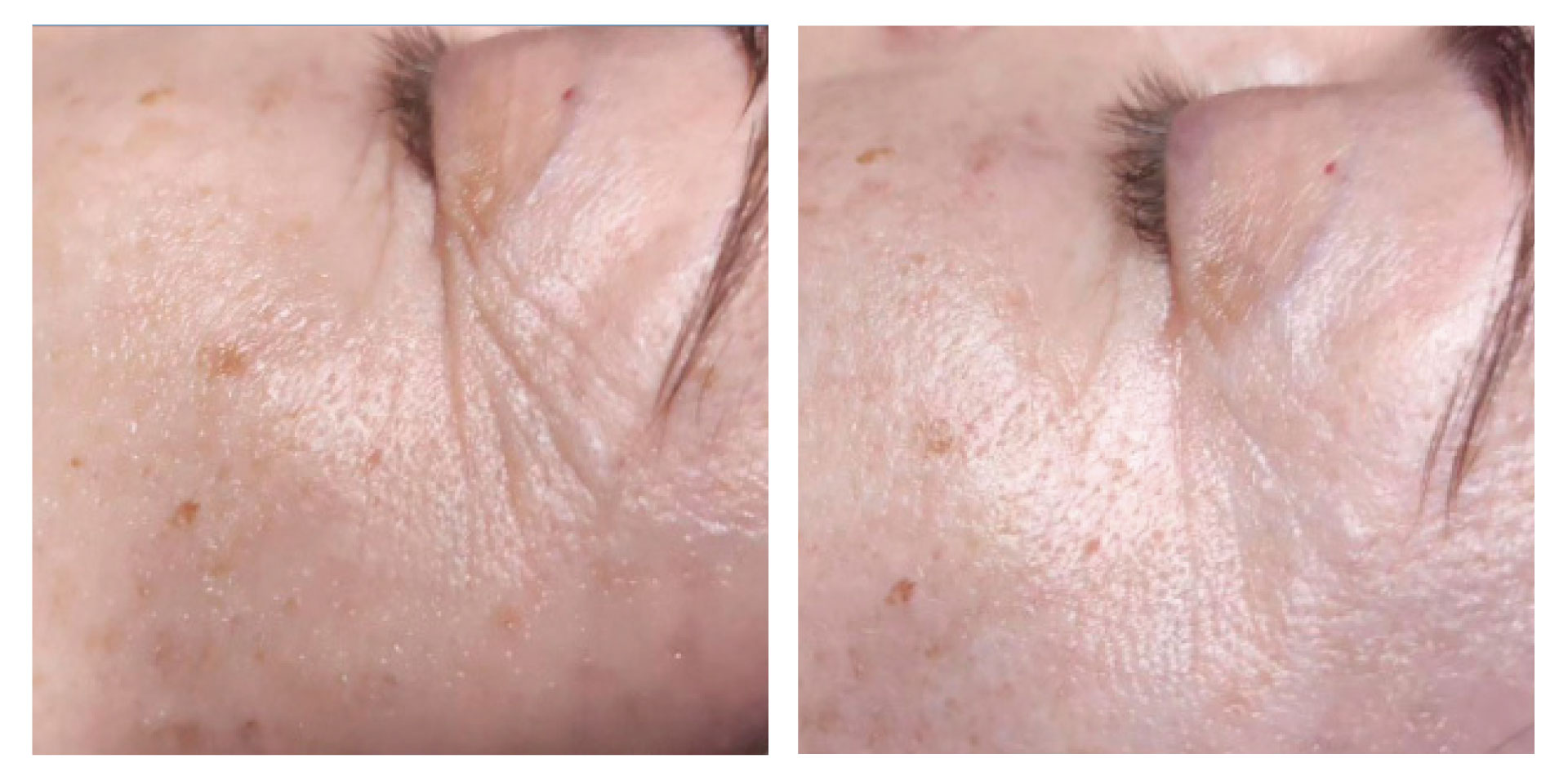
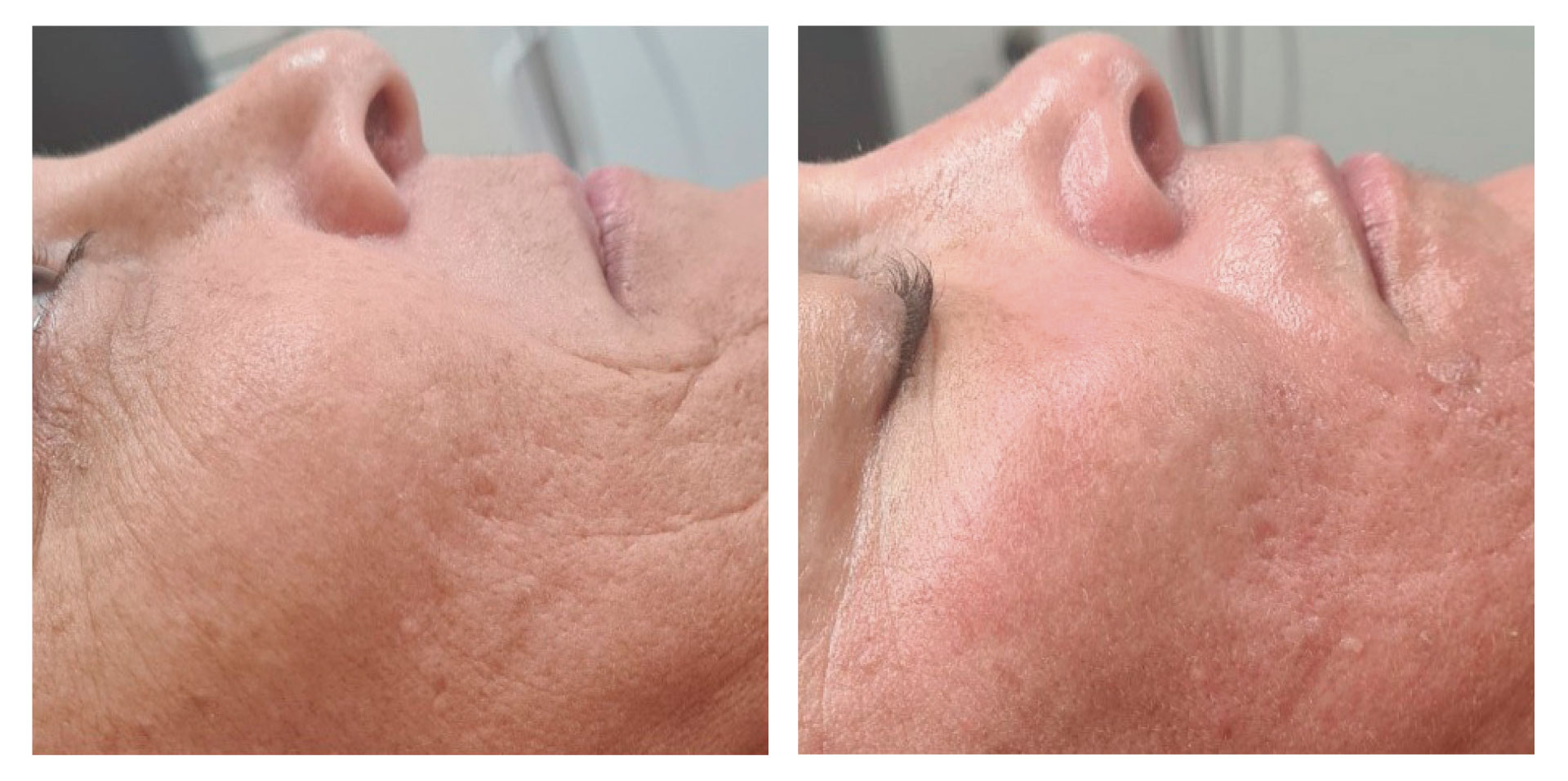
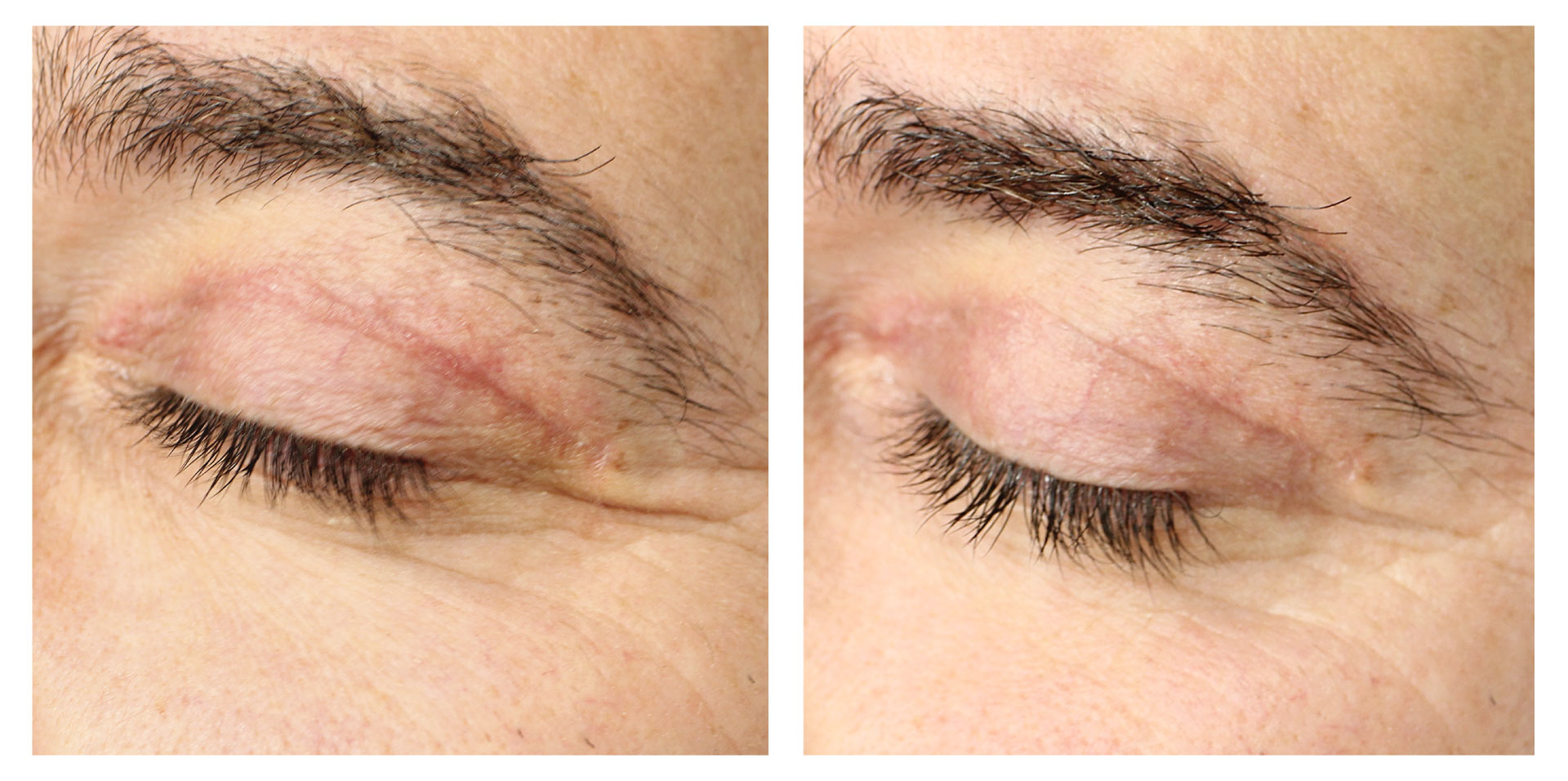
At the follow-up visit, significant reductions of 2% in body circumference and 9% in fat thickness were detected in the abdomen/flanks. These effects were accompanied by a significant 12% increase in dermal echogenicity, related to skin collagen content and organization. Furthermore, the blind evaluation of the photographic results revealed an overall visual improvement. A high satisfaction level was reported by the participants and no severe adverse events were detected.
Conclusion
Symmed treatment is a noninvasive procedure treating skin laxity and reducing abdomen/flanks circumferences safely and effectively.
Clinical Trial Registration
This clinical trial is not registered in a publicly accessible database, and no clinical trial registration number is available. At the time of the investigation, the intended use for fat reduction through radiofrequency was not classified as a medical application under European legislation (COUNCIL DIRECTIVE 93/42/EEC). In accordance with Spanish and European regulations, only clinical trials evaluating medical devices are required to be registered.
